Molecular Geometry And Electron Geometry Chart
Three in a plane with bond angles of 120 and two on opposite ends of the molecule.
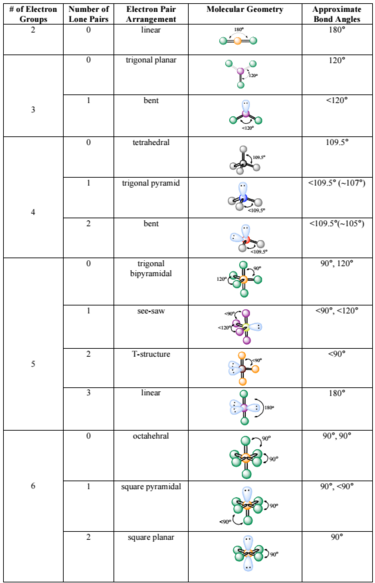
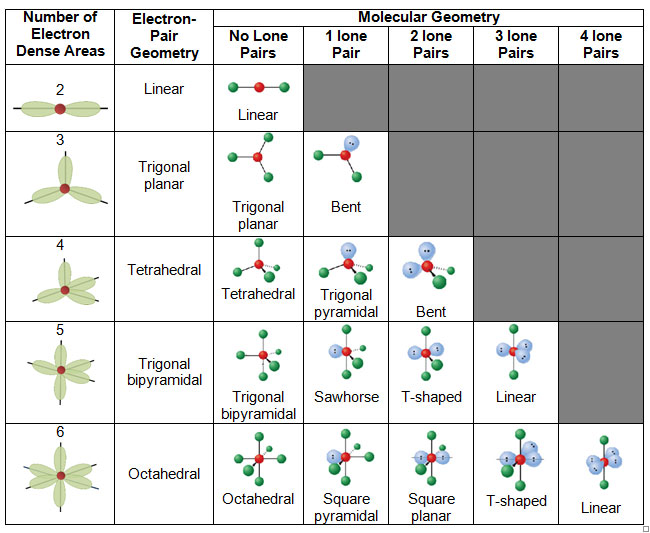
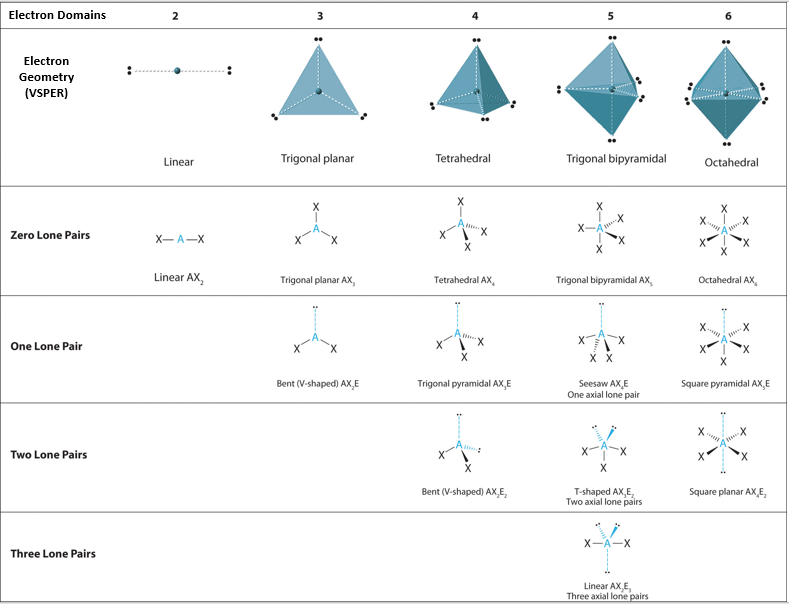
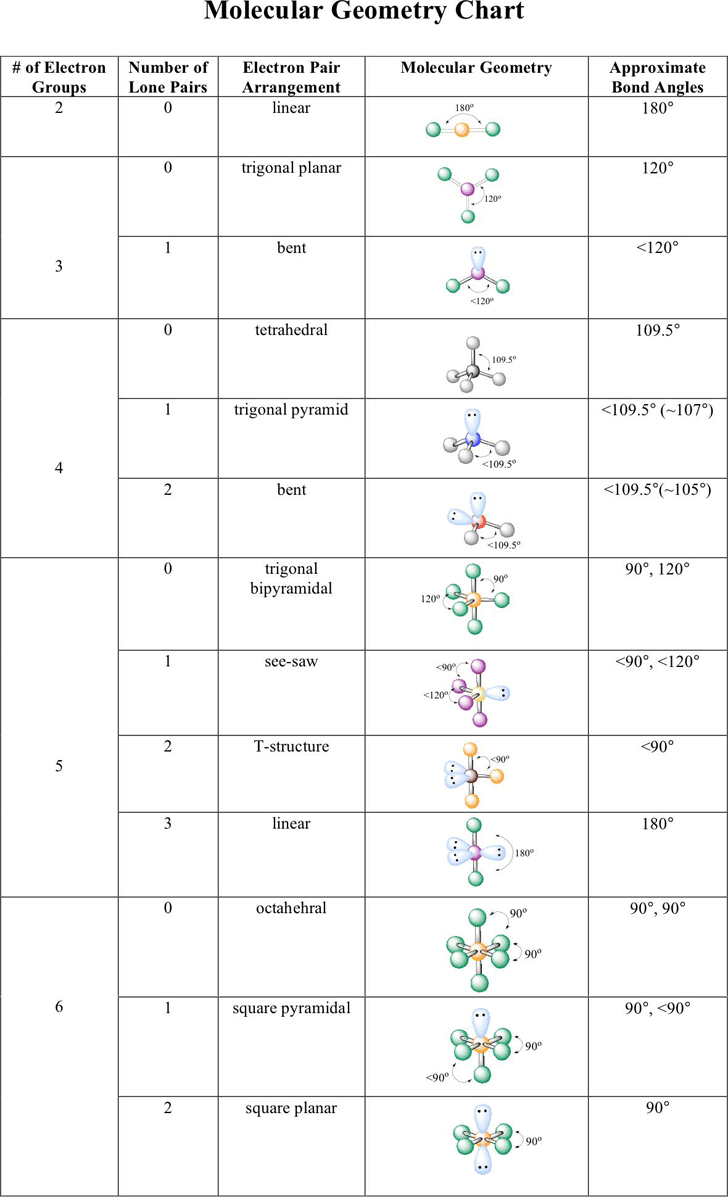
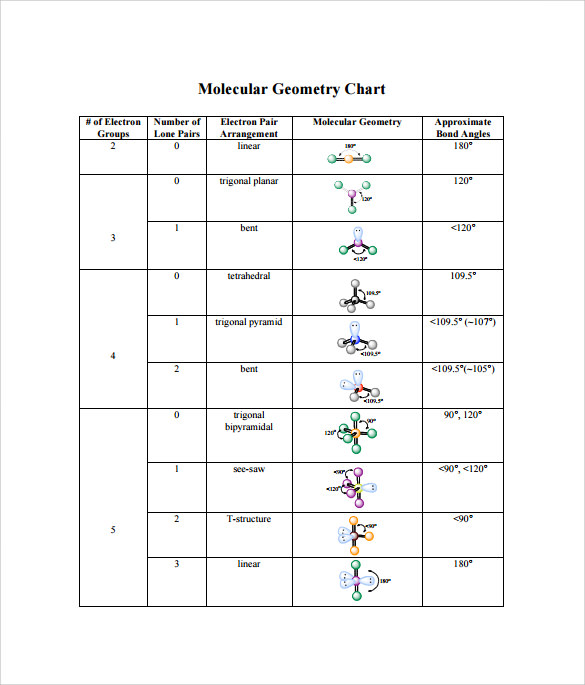
Molecular geometry and electron geometry chart. Four bonds on one central atom with bond angles of 1095. 14 rows For trigonal pyramidal geometry the bond angle is slightly less than 1095 degrees. Molecular Geometry from Trigonal Planar Electron Domain Geometry AB 2 E.
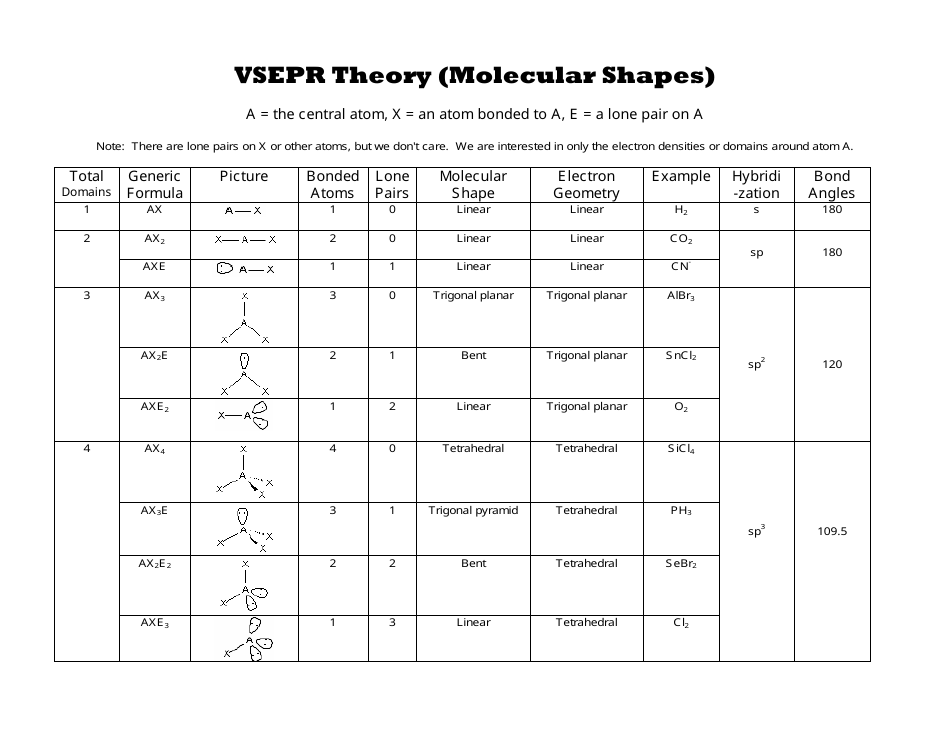
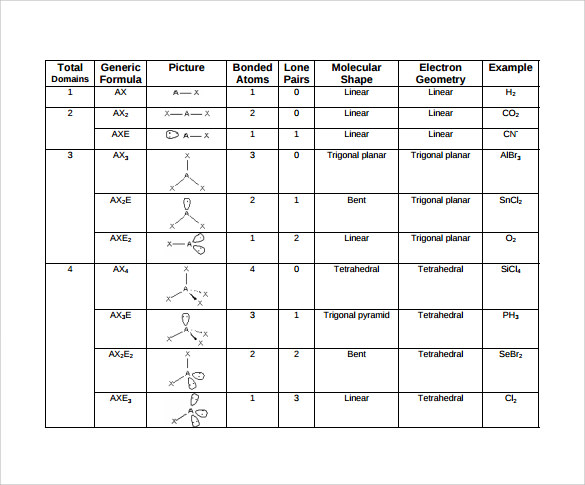
AX n E n 1. Molecular Geometry Van KoppenOffen Procedure. Electron Geometry is the arrangement of electron pairs around a central atom.
After the rope moves it pushes the molecules of the road. It is also irrational to say that an atom will cease to exist later. Molecular Electronic Geometry Chart Chemistry World Valence Molecular Geometry Chart Printable Pdf Download Math Shapes Graphing Worksheet I Can Graph Molecular Shape And Electron Domain Google Search Education Molecular Shapes Bagikan Artikel ini.
Each nucleus is composed of protons and neutrons. Molecular Orbital MO Diagram of O2. SO2 is an AX2E type molecule with 2 surrounding atoms ie oxygen and 1 lone pair of sulfur.
Seesaw T-shaped Linear Octahedral Square pyramidal Square planar Trigonal bipyramidal Trigonal bipyramidal. In CO32- ion we have one carbon atom and three oxygen atoms along with two negatively charged electrons carrying the charge. 1 2 3 0 1 2.
The molecular orbital diagram shows the energy state at each level where the excited state increases from the bottom to the top. Number of Electron Domains Electron-Domain Geometry Bonding Domains. It excludes lone pairs in deciding the shape of a molecule although repulsion from lone pair is taken into account only in bond angles.
Lone electron pairs are considered when finding the. Electron Domains Molecular Geometry and Bond Angles Hybridization of the Central atom linear 2 sp trigonal planar 3 sp2 tetrahedral 4 sp3 trigonal bipyramidal 5 sp3d octahedral 6 3 sp d2 180 120 120 90 90 1095. These molecules are usually polar with the exception of.
Six atoms around the central atom all with bond angles of 90. But the electron geometry of SO2 is trigonal planar. Count the Total Number of Valence Electrons.
Draw Lewis Structure determine Steric Number SN Molecular Geometry and Hybridization SN of atoms bonded to the central atom plus of lone pairs on the central atom SN the effective number of electron pairs surrounding a central atom. Posting Lebih Baru Posting. The left-hand side diagram is of O2 at ground level whereas the right-hand side diagram is of rearranged electrons as per the Lewis structure within the O2 molecule.
Five atoms around the central atom. VSEPR and Molecular Geometry Tables VSEPR Model VALENCE-SHELL ELECTRON-PAIR REPULSION VSEPR MODEL Lewis structures show the two-dimensional distribution of atoms and electrons. Here A central atom X surrounding atoms and E the lone pairs.
Belum ada Komentar untuk Electron Geometry Chart Posting Komentar. You must be wondering about this new term right. A molecular geometry chart is a collection of rules on how molecules and electrons will connect and shape a molecule.
Electron-domain and Molecular Geometries for Five and six electron domains around a Central atom. AX n E 0. Bent start with AB 3 molecule trigonal planar and replace a B atom w.
XeO3 is a polar molecule. Hence Lewis Structure is also commonly called Electron Dot Structure. Electron Geometry Hybridization Molecular Geometry VSEPR class Approximate Bond Angles 2 2 0 Linear sp Linear AX 2 180 3 0 Trigonal Planar AX 3 2 1 Bent AX 2 E 4 0 Tetrahedral AX 4 3 1 Trigonal Pyramidal AX 3 E 2 2 Bent AX 2 E 2 120 4 1095 3 Trigonal Planar sp 2 Tetrahedral sp 3 Geometry Examples VSEPR Predicting Molecular Geometry.
Molecular geometry is the shape of a molecule predicted by considering only bond electron pairs. We can easily find out the molecular geometry of any compound using the given chart. Electron geometry is the shape of a molecule predicted by considering both bond electron pairs and lone electron pairs.
A molecule will be non-polar if all dipoles cancel out otherwise it will be polar. The molecular geometry of XeO3 is trigonal pyramidal and its electron geometry is tetrahedral. The total valence electron is available for drawing the XeO3 Lewis structure is 26.
If one s and one p orbital hybridize they form two sp hybrid. The molecular geometry or three-dimensional shape of a molecule or polyatomic ion can be determined using. Let us proceed to draw the most appropriate LS diagram of CO32- ion.
Molecular Geometry Chart. Its molecular geometry is trigonal pyramidal while its electron geometry is tetrahedral. To drive through the air you must push the air molecules in the way.
Lewis Structure Representation of Valence Electrons in a molecule. 5 4 3 2. If all Xs are the same this will be a non-polar molecule.
Molecular geometry is the science of representing molecules in a three-dimensional manner. Admin June 5 2018. The steric number of Xenon central atom in the XeO3 molecule is 4 thus it forms Sp 3 hybridization.
Among the challenges that use mechanical CMM is that. Students and scientists can use these charts to create three-dimensional diagrams that represent molecules.